 DUCTILITY
DUCTILITY
- A metal is ductile when it may be drawn out in tension without rupture.
- Wire drawing depends upon ductility for its successful operation.
- A ductile metal must be both strong and plastic
- With many materials ductility increase rapidly with heat.
- Is the property of a material which enables it to be drawn easily into wire form
- The percentage elongation and contraction of area, as determined from a tensile test are a good practical measures of ductility
- Ability to undergo permanent change in shape without rupture or loss of strength if any force applied.
MALLEABILITY
- The ability to be hammered or rolled out without cracking.
- Very few metals have good cold malleability, but most are malleable when heated to a suitable temperature
- The material that can be shaped by beating or rolling is said to be malleable.
ELASTICITY
- The elasticity of a metal is its power of returning to its original shape after deformation by force.
- The ability to return to the original shape or size after having been deformed or loaded.
- All strain in the stressed material disappears upon removal of the stress.
PLASTICITY
- The property of flowing to a new shape under pressure/stress and retaining on the new shape after removal of pressure/stress.
- This is a rather similar property to malleability, and involves permanent deformation without rupture.
- It is opposite to elasticity
- The ability to deform permanently when load is applied.
Modulus of Elasticity E defined as the ratio of tensile stress to strain and determined in a tensile test.
Modulus of Rigidity G defined as the ration of shear stress and strain and determined in a torsion test.
Bulk Modulus K defined as the ration of pressure and volumetric strain and found with specialised equipment for liquids.
Poisson’s ratio ν defined as the ratio of two mutually perpendicular strains and governs how the dimensions of a material change such as reduction in diameter when a bar is stretched.
TOUGHNESS
- Resistance to fracture by blows.
- The materials usually have high tenacity combined with good or fair ductility.
- Toughness decreases with heating.
- A combination of strength and the ability to absorb energy or deform plastically.
- A condition between brittleness and softness.
- A materials ability to sustain variable load conditions without failure..
- Materials could be strong and yet brittle but a material is tough has strength
HARDNESS
- The hardness of a metal is a measure of its ability to withstand scratching, wear and abrasion, indentation by harder bodies, etc.
- The machine ability and inability to cut are also hardness property which is important for workshop process.
- Hardness also decreased by heating
- A material’s resistance to erosion or wear will indicate the hardness of the material
- A material’s ability to resist plastic deformation usually by indentation
HARDNESS MATERIALS LIST:
Hard materials are diamonds and glass. Soft materials are copper and lead. Hardness is measured by comparing it to the hardness of natural minerals and the list is called the Moh scale. The list runs from 1 to 10 with 1 being the softest ands 10 the hardest.
BRITTLENESS
- Opposite of toughness.
- A brittle material breaks easily under a sharp blow, although it may resist a steady load quite well.
- Brittle materials are neither ductile or malleable, but they often have considerable hardness.
- As a lack of ductility
- Strong materials may also be brittle
STIFFNESS/RIGIDTY
– This is the property of resisting deformation within the elastic range and for ductile materials is measured by the Modulus of Elasticity. A high E value means that there is a small deformation for a given stress.
– The property of a solid body to resist deformation, which is sometimes referred to as rigidity.
Strength
- The greater the load which can be carried the stronger the material and strength of the material will be higher.
- Tensile strength
- This is the main single criterion with reference to metals.
- This is the ability of a material to withstand tensile loads without rupture when the material is in tension
- It is a measure of the material’s ability to withstand the loads upon it in service.
- If the material is ductile, we look for the point at which it starts to stretch like a piece of plasticine. This point is called the yield point and when it stretches in this manner, we call it PLASTIC DEFORMATION.If the material is not ductile, it will snap without becoming plastic. In this case, we look for the stress at which it snaps and this is called the ULTIMATE TENSILE STRENGTH.Most materials behave like a spring up to the yield point and this is called ELASTIC DEFORMATION and it will spring back to the same length when the load is removed.
Ultimate tensile strength (UTS)
(Tensile strength or Ultimate Strength)
– It is the maximum stress that a material can withstand while being stretched or pulled before failing or breaking. Tensile strength is not the same as compressive strength and the values can be quite different.
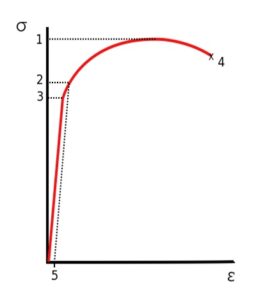
– UTS is usually found by performing a tensile test and recording the engineering stress versus strain. The highest point of the stress-strain curve (see point 1 on the engineering stress/strain diagrams below) is the UTS.
Stress vs. Strain curve typical of aluminum.
1 Ultimate Strength
2 Yield Strength
3 Proportional Limit Stress
4 Rupture
5 Offset Strain (usually 0.002)
Compressive Strength
This is the ability of a material to withstand Compressive (squeezing) loads without being crushed when the material is in compression.
Shear Strength
This is the ability of a material to withstand offset or traverse loads without rupture occurring.
Fatigue Strength
This is the property of a material to withstand continuously varying and alternating loads.
Yeild Strength
The stress a material can withstand without permanent deformation.
Torsional Strength
This governs the stress at which a material fails when it is twisted and a test
similar to the tensile test is carried out, only twisting the specimen instead of
stretching it. This is a form of shearing.
HEAT TREATMENT
Heat treatment is a general term referring to a cycle of heating and cooling which alters the internal structure of metal and thereby changes its properties
- Metal and alloys are heat treated for a number of purposes however the primarily to:-
- Increase their hardness and strength
- To improved ductility
- To soften them for subsequent operations (cutting etc)
- Stress relieving
- Eliminate the effects of cold work
HEAT TREATMENT OF STEEL
The mechanical properties of materials can be changed by heat treatment. Let’s first examine how this applies to carbon steels.
CARBON STEELS
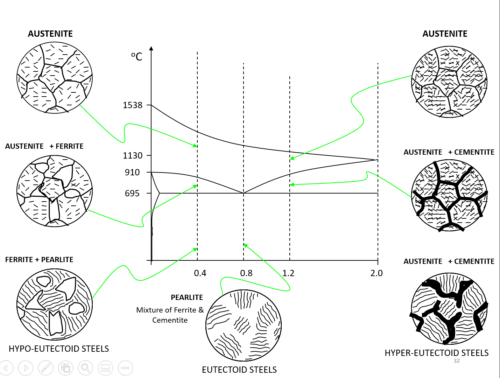
– In order to understand how carbon steels are heat-treated we need to re-examine the structure. Steels with carbon fall between the extremes of pure iron and cast iron and are classified as follows.
– All metals form crystals when they cool down and change from a liquid into a solid. In carbon steels, the material that forms the crystals is complex. Iron will chemically combine with carbon to form IRON CARBIDE (Fe3C). This is also called CEMENTITE. It is white, very hard and brittle. The more cementite the steel contains, the harder and more brittle it becomes.
– When it forms in steel, it forms a structure of 13% cementite and 87% iron (ferrite) as shown. This structure is called PEARLITE. Mild steel contains crystals of iron (ferrite) and pearlite as shown. As the % of carbon is increased, more pearlite is formed and at 0.9% carbon, the entire structure is pearlite.
AUSTENITE (γ)
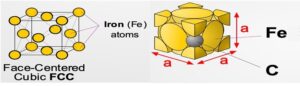
- At high temperatures (i.e. roughly>712°C ), iron can form in a Face-Centred Cubic (FCC) structure called austenite, or γ-austenite.
- repeating crystal unit cell is also equal in length on all sides but has a Fe atom at the center of each face, as well as 8 Fe atoms at its vertices. Unlike FCC α ferrite, there is no atom at the center of the γ -austenite unit cell.
- A solid solution of Carbon in face-centered cubic iron, containing a maximum 0f 1.7 % carbon at 1130oC
- It is soft, ductile and non-magnetic and also exists in the plain carbon steel above the upper critical range.
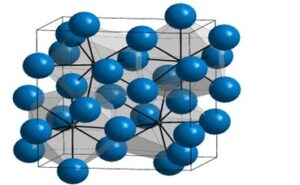
 FERRITE (α)
FERRITE (α)
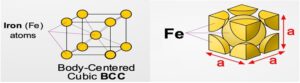
- Given enough time, and at low temperatures (i.e. roughly <912°C ), iron can form is in a Body-Centred-Cubic (BCC) structure commonly referred to as ferrite, or α-ferrite.
- Repeating crystal unit cell is equal in length on a side and has a single Fe atom at its center, and 8 Fe atoms at its vertices.
- Ferrite is nearly pure iron. A solid solution of Carbon in body-centered cubic (BCC) iron, containing a maximum of 0.04 % Carbon at 695oC.
- It is the softest constitute of steel and very ductile and readily cold-worked
 CEMENTITE
CEMENTITE
- Cementite (also known as Iron Carbide, or Fe3C is a stable iron-carbon compound that forms when no further diffusion of carbon into α-ferrite or γ-austenite is possible.
- Cementite contains up to 6.67% carbon. Because of its increased carbon content, cementite is hard and brittle, and its presence is usually a byproduct, rather than by design.
- Cementite commonly occurs in steels when excess carbon, such as left-over carbon which cannot be absorbed into ferrite, must be used for the formation of crystals.
PEARLITE
- pearlite is a layered metallic structure of two phases, which compose of alternating layers of ferrite (87.5%) and cementite (12.5%) that occurs in some steels and cast irons.
- It contains 0.83% Carbon and is formed by the breakdown of the austenite solid solution at 695oC
- The properties of pearlite are harder and stronger than ferrite, but softer and more ductile than cementite
MARTENSITE:
- Martensite is formed in steels when the cooling rate from austenite is sufficiently fast.
- It is a very hard constituent, due to the carbon which is trapped in a solid solution.
- For steel 0–0.6% carbon the martensite has the appearance of lath and is called lath martensite. For steel greater than 1% carbon it will form a plate-like structure called plate martensite
- Martensite is produced from austenite as a result of the quenching, or another form of rapid cooling from a temp. range 1500 – 723°C
BAINITE:
- Bainite is a plate-like microstructure that forms in steels from austenite when cooling rates are not rapid
- Bainite is a type of steel that’s produced by cooling faster than pearlite but slower than martensite.
- Bainite is often preferred because it doesn’t require tempering after being hardened.
- in the temperature range 250–375 °C both upper and lower bainite could be seen, simultaneously
- If the carbon is increased further, more cementite is formed and the structure becomes pearlite and cementite.
 Hypo-eutectoid steel:
Hypo-eutectoid steel:
- Steel with a carbon fraction less than C = 0.76% is called a hypo-eutectoid steel. It is located left of the eutectoid point in the diagram.
Hypereutectoid steel:
- Steels with a larger fraction of carbon are called hypereutectoid steels.
HEAT TREATMENT of CARBON STEELS
Steels containing carbon can have their properties (hardness, strength, toughness etc) changed by heat treatment. Basically, if it is heated up to red hot and then cooled very rapidly the steel becomes harder. Dead mild steel is not much affected by this but a medium or high carbon steel is.
Principle of heat treatment of steel
- Metals are never heated to the melting point in heat treatment.
- Therefore, all the reactions within the metal during the heating and cooling cycle, take place while the metal is in the solid state
- During ordinary heat treating operations, steel is seldom heated above 983oC.
- In using the iron-iron carbide diagram, we need only to concern ourselves with that part which is always solid steel.
- The area where the Carbon content is 2% or less and the temperature is below 1130oC
COOLING RATE
- Cooling rate is the most important part of heat treatment.
- Different cooling rates are now considered as they have a significant effect on the properties of the metal.
SLOW COOLING
- Austenite is transformed to course pearlite.
- Slightly more rapid cooling may produce fine pearlite in which the layers of ferrite and cementite are thinner.
INTERMEDIATE COOLING
- Austenite transforms to a material called Bainite instead of the usual pearlite.
- When etched, Bainite gives a dark appearance and shows a circular or needle like form.
FAST COOLING
- By quenching in water, the transformation of austenite is suppressed until about 318oC at which point a new constituent called Martensite(quite brittle) begins to form instead of the Bainite or pearlite of slower cooling rate.
- As the temperature drops lower, the transformation become complete.
- This temperature vary with the alloy content of the steel
QUENCHING
- To harden by quenching, a metal (usually steel or cast iron) must be heated into the austenitic crystal phase and then quickly cooled.
Quenching Media:
- Brine (water and salt solution)
- Water
- Oil
- Air
- Turn off the furnace
TIME-TEMPERATURE TRANSFORMATION
- In order to obtain steels with the desired properties, we must have some control over the transformation process, and this is indicated in the TTT diagram
- TTT diagrams are used to predict the metallurgical structure of a steel sample which is quenched in the austenite region and held to a constant elevated temperature below 729oC.
- This is known as Isothermal transformation
However since heat treatment usually involves continuous cooling, TTT diagrams are not directly applicable but can be modified to be useful in at least a qualitative way for continuous cooling condition
Heat treatment Methods
Annealing
- heat treatment that alters the microstructure of a material causing changes in properties such as strength, hardness, and ductility
- It the process of heating solid metal to high temperatures and cooling it slowly so that its particles arrange into a defined lattice
Stages in annealing
- Heating to the desired temperature ,
- Holding or soaking at that temperature,
- Cooling or quenching,usually to room temperature .
In practice annealing concept is most widely used in heat treatment of iron and steals
Purpose of annealing
It is used to achieve one or more of the following purpose.
- To relive or remove stresses
- To include softness
- To alter ductility, toughness, electrical, magnetic.
- To Refine grain size
- To remove gases
- To produce a definite microstructure.
Application
Annealing process is employed in following application
- Casting
- Forging
- Rolled stock
- Press work ….
3 Types of Annealing:
- Process Annealing
- Full annealing
- Spheroidising
1. Process Annealing
- Carried out on cold-worked low carbon steel sheet or wire in order to relieve internal stress and to soften the metals.
- The steel is heated to 550 to 650oC below the critical point.
2. Full Annealing
- It carried out on hot-worked and cast steels in order to obtain grain refinement with high ductility.
- It also produces a softer steel with better machinability
- For steels
- heating above critical point (30 – 50oC) then
- holding at this temperature for a time (thickness) – followed by slow cooling usually in furnace.
3. Spheroidizing Annealing
- To remove coarse pearlite and making machining process easy .
- It forms spherodite structure of maximum soft and ductility easy to machining and deforming.
- The process is limited to steels in excess of 0.5% carbon. This steel can be softened by annealing at 650 – 750oC just below the lower critical point, when the cementite of the pearlite balls up or spheroidizes.
Defects uncontrolled temperature
Heated above the actual temperature or to long maintained at annealing temperature: austenite grain growth will occur and make the metal weak and brittle
If heated above the upper critical point to temperature, Brittles films of oxide are formed which make the steel unsuitable. For further use and must be remelted.
The original pearlite will have change to several small austenite grains.
NORMALIZING
- For hypoeutectoid steels
- heating above critical point (30 – 50oC)
- holding at this temperature for a time (thickness) &
- followed by cooling in still air.
- Produces maximum grain refinement and consequently the steel slightly harder and stronger than a fully annealed steel.
- However the properties will vary with section thickness
HARDENING
- Hardening is process in which Medium and High carbon steels (0.4 – 1.2%) is heated to a temperature above the critical point (until red hot), held at this temperature and quenched (rapidly cooled) in water, oil or molten salt baths.
- Hardening producing a very hard and brittle metal. At 723 Deg C, the ferrite changes into Austenite structure.
TEMPERING
- Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys.
- To remove some of the brittleness from hardened steels, tempering is used. The metal is heated to the range of 220-300 deg C and cool in the air.
- Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical temperature for a certain period of time, then allowed to cool in still air.
THE AFFECT OF PROCESSING and MANIPULATION ON METALS
When a metal solidifies grains or crystals are formed. The grains may be small, large or long depending on how quickly the material cooled and what happened to it subsequently. Heat treatment and other processes carried out on the material will affect the grain size and orientation and so dramatically affect the mechanical properties. In general slow cooling allows large crystals to form but rapid cooling promotes small crystals. The grain size affects many mechanical properties such as hardness, strength and ductility.
MANIPULATIVE PROCESSES
These are processes which shape the solid material by plastic deformation. If the process is carried out at temperatures above the crystallisation temperatures, then re-crystallisation occurs and the process is called HOT WORKING. Otherwise the process is called COLD WORKING. The mechanical properties and surface finish resulting are very different for the two methods.
HOT ROLLING
This is used to produce sheets, bars and sections. If the rollers are cylindrical, sheet metal is produced. The hot slab is forced between rollers and gradually reduced in thickness until a sheet of metal is obtained. The rollers may be made to produce rectangular bars, and various shaped beams such as I sections, U sections, angle sections and T sections. Steel wire is also produced this way. The steel starts as a round billet and passes along a line of rollers. At each stage the reduction speeds up the wire into the next roller. The wire comes of the last roller at very high speeds and is deflected into a circular drum so that it coils up. This product is then used for further drawing into rods or thin wire to be used for things like springs, screws, fencing and so on.
COLD ROLLING
The process is similar to hot rolling but the metal is cold. The result is that the crystals are elongated in the direction of rolling and the surface is clean and smooth. The surface is harder and the product is stronger but less ductile. Cold working is more difficult that hot working.
FORGING
In this process the metal is forced into shape by squeezing it between two halves of a die. The dies may be shaped so that the metal is simply stamped into the shape required (for example producing coins). The dies may be a hammer and anvil and the operator must manipulate the position of the billet to produce the rough shape for finishing (for example large gun barrels).
COLD WORKING
Cold working a metal by rolling, coining, cold forging or drawing leaves the surface clean and bright and accurate dimensions can be produced. If the metal is cold worked, the material within the crystal becomes stressed (internal stresses) and the crystals are deformed. For example cold drawing produces long crystals. In order to get rid of these stresses and produce “normal” size crystals, the metal can be heated up to a temperature where it will re-crystallise. That is, new crystals will form and large ones will reduce in size.
If the metal is maintained at a substantially higher temperature for a long period of time, the crystals will consume each other and fewer but larger crystals are obtained. This is called “grain growth”.
Cold working of metals change the properties quite dramatically. For example, cold rolling or drawing of carbon steels makes the stronger and harder. This is a process called “work hardening”.
HOT WORKING
Most metals (but not all) can be shaped more easily when hot. Hot rolling, forging, extrusion and drawing is easier when done hot than doing it cold. The process produces oxide skin and scale on the material and producing an accurate dimension is not possible.
Hot working, especially rolling, allows the metal to re-crystallise as it is it is produced. This means that expensive heat treatment after may not be needed. The material produced is tougher and more ductile.
LIQUID CASTING AND MOULDING
When the metal cools it contracts and the final product is smaller than the mold. This must be taken into account in the design.
The mold produces rapid cooling at the surface and slower cooling in the core. This produces a different grain structure and the casting may be very hard on the outside. Rapid cooling produces fine crystal grains. There are many different ways of casting.
SAND CASTING
A heavy component such as an engine block would be cast in a split mould with sand in it. The shape of the component is made in the sand with a wooden blank. Risers allow the gasses produced to escape and provide a head of metal to take up the shrinkage. Without this, the casting would contain holes and defects.
Sand casting is an expensive method and not ideally suited for large quantity production. Typical metals
used are cast iron. Cast steel and aluminum alloy.
DIE CASTING
Die castings uses a metal mould. The molten metal may be fed in by gravity as with sand casting or forced in under pressure. If the shape is complex, the pressure injection is the best to ensure all the cavities are filled. Often several moulds are connected to one feed point. The moulds are expensive to produce but this is offset by the higher rate of production achieved. The rapid cooling produces a good surface finish with a pleasing appearance. Good size tolerance is obtained. The best metals are ones with a high degree of fluidity such as zinc. Copper, aluminium and magnesium with their alloys are also common.
CENTRIFUGAL CASTING
This is similar to die casting. Several moulds are connected to one feed point and the whole assembly is rotated so that the liquid metal is forced into the moulds. This method is especially useful for shapes such as rims or tubes. Gear blanks are often produced this way.
MACHINING
Machining processes involve the removal of material from a bar, casting, plate or billet to form the finished shape. This involves turning, milling, drilling, grinding and so on. Machining processes are not covered in depth here. The advantage of machining is that is produces high dimensional tolerance and surface finish which cannot be obtained by other methods. It involves material wastage and high cost of tooling and setting.
CASE HARDENING
- Low carbon steels cannot be hardened by heating due to the small amounts of carbon present. So, Case hardening seeks to give a hard outer skin over a softer core on the metal.
- The addition of carbon to the outer skin is known as carburizing.
- Low carbon steels cannot be hardened by heating due to the small amounts of carbon present. So, Case hardening seeks to give a hard outer skin over a softer core on the metal.
Carburizing
- The addition of carbon to the outer skin is known as carburizing.
ALLOYS
Nickel
– One of the most widely used alloying elements in steel. In amounts 0.50% to 5.00% its use in alloy steels increases the toughness and tensile strength without detrimental effect on the ductility.
Chromium
– Gives resistance to wear and abrasion. Chromium has an important effect on corrosion resistance and is present in stainless steels in amounts of 12% to 20%.
Molybdenum
– Increases hardenability, toughness to quenched/tempered steels. It also improves the strength of steels at high temperatures (red- hardness).
Vanadium
– Steels containing vanadium have a much finer grain structure than steels of similar composition without vanadium.
CREEP
- Creep is strain increase with time under constant load.
- Creep is temperature dependent – the higher the temperature the greater the effect
FRETTING
A type of wear that occurs between tight-fitting surfaces subjected to cyclic relative motion of extremely small amplitude. Usually, fretting is accompanied by corrosion, especially of the very fine wear debris.
 DUCTILITY
DUCTILITY
 FERRITE (α)
FERRITE (α)
 CEMENTITE
CEMENTITE
 Hypo-eutectoid steel:
Hypo-eutectoid steel: